lisaleaks
~ Literary Agent Investigative Journalist
Life Obeys Physical Laws
17SaturdayAug 2013
Damn Interesting!
Science tells us that the laws of physics are not subject to change, and that these laws determine the interaction of all matter and energy in existence. If no matter or energy– at any level– is outside of the influence of these immutable laws, it could be suggested that every event which ever occurs is the inevitable result of a series of events which began at the inception of the universe.
For the sake of argument, let us suggest that there is no true randomness in the universe, only unpredictability. This would mean that all of the matter and energy which was ejected from the Big Bang followed a set path as the universe expanded. Each particle went where the laws of physics determined it must, depending on its inertia, gravitational influence, friction, etc.
Every object that ever existed was formed by the interaction of matter and energy, working within these laws. Consequently, stars, planets, puppies, and brown paper packages tied up with string all exist because each particle in the collection of particles that make them up followed the laws of physics, from the moment they sprang into existence until the moment they came together to form the object. Furthermore, the path that physics set these particles upon will eventually lead them elsewhere, where they will contribute to the temporary existence of some other inevitable assembly of particles. If it were possible to observe every particle in the universe at once, and one had a complete knowledge of the laws of physics, the future would not be a mystery, it would be predictable with 100% accuracy.
Clearly, this website is the unavoidable product of the creation of the universe. It had to spring into existence at exactly the moment it did. The laws of physics demanded it.
Matter and Energy Laws
Life obeys physical laws.
Drink some water, eat some food, run to class. The two things that connect these activities and other aspects of life on earth are matter and energy. Matter has mass and occupies space: it is the stuff you and everything else is made of.
Matter comes in a variety of forms. We call these different unique types of matter elements. An atom is the smallest unit of an element that has all of the properties of that element. There are 92 naturally occurring elements in nature. These different forms of matter differ uniquely in their physical and chemical properties: carbon (C) and hydrogen (H) differ in their size, reactiveness with other atoms, and other physical and chemical properties. An element is a substance that cannot be broken down to other substances by ordinary chemical means. An element can be combined with another to make acompound. For instance, hydrogen combines with oxygen to produce water. Scientists use symbols (hydrogen=H, oxygen=O) as a kind of short-hand for describing compounds. For example H2O is mean water is comprised of 2 toms of hydrogen and one atom of oxygen.
Energy is a more elusive concept. Formally, it is defined as the ability (or capacity) to do work Work is the product of force and distance. When you are walking up the Hill, you are doing work by applying muscles (force) to move up the Hill (distance). Energy is what you and all living things use to move matter around and to change matter from one form to another. Energy is used to grow your food, to keep you alive (metabolism), to move you from one place to another, and to warm and cool the buildings in which you work and live. The uses and transformations of matter and energy are governed by certain scientific laws, which unlike the laws people enact, cannot be broken.
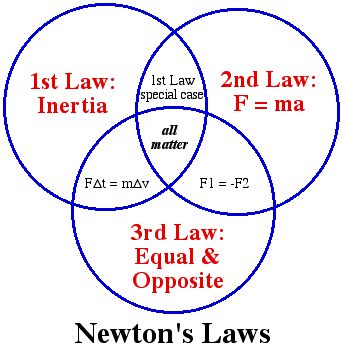
III. There are three physical laws governing matter and energy that are important to us.
B) First law of energy (first law of Thermodynamics)
C) Second law of energy (second law of thermodynamics)
A. Law of Conservation of Matter: (everything must go somewhere)
We talk about consuming, or using up material resources, but actually we don’t consume any matter. We only borrow some of the earth’s resources for a while C taking them from the earth, carrying them to another part of the globe, processing them, using them, and then discarding, reusing, or recycling them. In the process of using matter we may change it to another form, but in every case we neither create nor destroy any measurable amount of matter. This results from the law of conservation of matter: In any physical or chemical change, matter is neither created nor destroyed but merely changed from one form to another. When you throw away something, remember there is no “away.” Everything we think we have thrown away is still here with us in one form or another.
How does this affect environmental science ? Although we can certainly make the environment cleaner, the law of conservation of matter says we will always be faced with pollution of some sort. This means that we must trade-off one form of pollution for another. This trade-off involves making controversial scientific, political, economic, and ethical judgments about what is a dangerous pollution level, to what degree a pollutant must be controlled, and what amount of money we are willing to pay to reduce the amount of a pollutant to a harmless level.
B. The First Law of Energy (First Law of thermodynamics): You can’t get something for nothing
We talk about consuming, or using up material resources, but actually we don’t consume any matter. We only borrow some of the earth’s resources for a while C taking them from the earth, carrying them to another part of the globe, processing them, using them, and then discarding, reusing, or recycling them. In the process of using matter we may change it to another form, but in every case we neither create nor destroy any measurable amount of matter. This results from the law of conservation of matter: In any physical or chemical change, matter is neither created nor destroyed but merely changed from one form to another. When you throw away something, remember there is no “away.” Everything we think we have thrown away is still here with us in one form or another.
How does this affect environmental science ? Although we can certainly make the environment cleaner, the law of conservation of matter says we will always be faced with pollution of some sort. This means that we must trade-off one form of pollution for another. This trade-off involves making controversial scientific, political, economic, and ethical judgments about what is a dangerous pollution level, to what degree a pollutant must be controlled, and what amount of money we are willing to pay to reduce the amount of a pollutant to a harmless level.
B. The First Law of Energy (First Law of thermodynamics): You can’t get something for nothing
You encounter energy in many forms: mechanical, chemical (food and fuel), electrical, nuclear, heat, and radiant (such as light). Scientists usually classify most forms of energy as either potential or kinetic energy.
1) Kinetic energy is the energy that matter has because of its motion and mass. A moving car, falling rock, and the flow of electrons or charged particles called electrical energy are all examples of kinetic energy.
The amount of kinetic energy matter has depends on both its mass and its velocity (speed). Because of its velocity a bullet fired from a gun can cause more damage that one thrown by hand; and a bowling ball dropped on your foot does more damage that a pool ball.
2) Potential energy: The energy stored by an object as a result of its position or the position of its parts is called potential energy. A rock held in your hand, a bowl of cereal, a stick of dynamite, and a tank of gas are all examples. The rock has stored (or potential) energy that can be released and converted into kinetic energy (in the form of mechanical energy and heat) if it is dropped. Doing work involves changing energy from one form to another.
- When you lift an object, chemical energy (a form of potential energy) stored in the chemicals obtained from your digested food is converted into the mechanical energy (kinetic) used to move your arm and the object upward and into heat given off by your body
- In an automobile engine, the chemical energy stored in the gasoline is converted into mechanical energy that propels the car and is eventually lost as heat (engine heat), friction of the tires with the ground, and energy imparted to the air as it is pushed out of the way by your car.
- A battery converts chemical energy into electrical energy andheat (low grade form of kinetic energy.
- In an electric power plant, chemical energy from fossil fuels (potential) or nuclear energy from uranium nuclear fuel (potential) is converted into a combination of mechanical energy and heat. The mechanical energy is used to spin the turbine that converts the mechanical energy into electrical energy and more heat. When the electrical energy oscillates through the filament wires in an ordinary light bulb, it is converted into light and still more heat. Note that in all of these transformations, some energy is always lost as heat that flows into the surrounding environment.
3) Energy changes: What energy changes occur when you drop a rock? Because of its higher position, the rock in your hand has a higher potential energy than the same rock at rest on the ground. When you drop the rock and it hits and eventually rests on the ground, the rock now has a much lower potential energy. Has the amount of energy changed (i.e., the rock lost energy – where did it go?) At first glance it seems so. But according to the first law of conservation of energy, in any ordinary physical or chemical process is neither created nor destroyed but merely change from one form to another.
The energy lost by a system or collection of mater under study (in this instance, the rock) must equal the energy gained by the surroundings or environment (in this instance, air molecules pushed out of the way, and soil particles moved by the impact of the rock). This energy law holds for all systems, living and nonliving.
Let’s look at what really happens. As the rock drops, its potential energy is changed into kinetic energy C both its own and that of the air through which it passes. The friction created when the rock is drops through the air causes air molecules in the air to move faster, so their average temperature rises. This means that some of the rock’s original potential energy has been transferred to the air as heat. When the rock hits the ground more of its mechanical energy is transferred to particles of soil. The energy lost by the rock (system) is exactly equal to the energy gained by its surroundings. Scientists have never seen an instance where energy input does not equal energy output.
C. Second Law of Energy (Second law of thermodynamics): You can’t break even
Energy quality: Because according to the first energy law energy can neither be created nor destroyed, you might think there will always be enough energy. Yet when you fill a car’s tank with gasoline and drive around something is lost. If it isn’t energy, what is it? The second law of energy, also known as the second law of thermodynamics provides the answer to this question.
Energy varies in its quality or ability to do useful work. For useful work to occur energy must move or flow from a level of high-quality (more concentrated) energy to a level of lower-quality (less concentrated) energy. The chemical potential energy concentrated in a lump or coal or a tank of gasoline and the concentrated heat energy at a high temperature are forms of high-quality energy. Because the energy in gasoline or coal is concentrated, they have the ability to perform useful work in moving or changing matter . In contrast, less concentrated heat energy at a low temperature has little remaining ability to perform useful work.
Over the years, after investigating millions of conversions of energy from one form to another, scientists have found that some of the energy is always degraded to a more dispersed and less useful form, usually as heat given off at a low temperature to the surroundings.
Let’s look at what really happens. As the rock drops, its potential energy is changed into kinetic energy C both its own and that of the air through which it passes. The friction created when the rock is drops through the air causes air molecules in the air to move faster, so their average temperature rises. This means that some of the rock’s original potential energy has been transferred to the air as heat. When the rock hits the ground more of its mechanical energy is transferred to particles of soil. The energy lost by the rock (system) is exactly equal to the energy gained by its surroundings. Scientists have never seen an instance where energy input does not equal energy output.
C. Second Law of Energy (Second law of thermodynamics): You can’t break even
Energy quality: Because according to the first energy law energy can neither be created nor destroyed, you might think there will always be enough energy. Yet when you fill a car’s tank with gasoline and drive around something is lost. If it isn’t energy, what is it? The second law of energy, also known as the second law of thermodynamics provides the answer to this question.
Energy varies in its quality or ability to do useful work. For useful work to occur energy must move or flow from a level of high-quality (more concentrated) energy to a level of lower-quality (less concentrated) energy. The chemical potential energy concentrated in a lump or coal or a tank of gasoline and the concentrated heat energy at a high temperature are forms of high-quality energy. Because the energy in gasoline or coal is concentrated, they have the ability to perform useful work in moving or changing matter . In contrast, less concentrated heat energy at a low temperature has little remaining ability to perform useful work.
Over the years, after investigating millions of conversions of energy from one form to another, scientists have found that some of the energy is always degraded to a more dispersed and less useful form, usually as heat given off at a low temperature to the surroundings.
In an internal combustion automobile engine, only about 20% of the high-quality chemical energy available in the gasoline is converted to mechanical energy used to propel the car; the remaining 80% is degraded to low-quality heat that is released into the environment. In addition, about 50% of the mechanical energy produced is also degraded to low-quality heat energy through friction, so that 90% of the energy in gasoline is wasted and not used to move the car.
When electrical energy oscillates through the filament wires in an ordinary light bulb, it is converted into a mixture of about 5% useful radiant energy (light) and 95% low-quality heat.
It is interesting to note that much of modern civilization is built around the internal combustion engine and the incandescent light that, respectively, waste 90 and 95% of their initial energy input. Some of this waste is due to the energy-quality tax automatically exacted as a result of the second energy law and some is due to technological designs that waste more energy that necessary.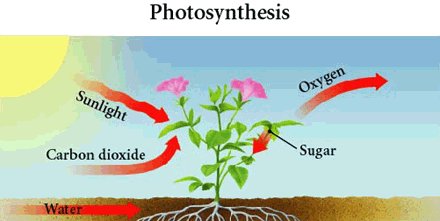

Most energy exchange processes occur like this (high quality energy to low quality) but there is one VERY IMPORTANT exception: the conversion of solar energy to chemical energy in food by plants and some bacteria. Photosynthesis converts radiant energy (light) from the sun into high-quality chemical energy (stored in the plant in the form of sugar molecules) and low-quality heat energy. If you eat plant food [like spinach], its high-quality chemical energy is transformed within your body to high-quality mechanical energy, used to move your muscles and to perform other life processes, and low-quality heat energy. The process of breaking down food such as sugars to simpler molecules, such as CO2 and water, releasing potential energy in the process, is called respiration. At each step, the low-quality heat flows into the environment. Without the action of plants and bacteria, life as we know it would not exist because animals have no way of turning the radiant energy from the sun into high energy (high quality) food.
So, the first energy law governs the quantity of energy available from an energy conversion process, whereas the second energy law governs the quality of energy available. According to the first law we will never run out of energy, but according to the second law we can run out of high quality or useful energy.
Not only can we not get something for nothing (the first law), we can’t even break even in terms of energy quality (the second law)
Not only can we not get something for nothing (the first law), we can’t even break even in terms of energy quality (the second law)
The second energy law also tells us that high-grade energy can never be used over again.
We can recycle matter but we can never recycle high-quality energy.
Fuels and foods can be used only once to perform useful work. Once a piece of coal or a tank full of gasoline is burned, its high-quality potential energy is lost forever. This means that the net useful, or high-quality energy available from fossil fuels, uranium, or any concentrated energy source is even less than predicted by the first energy law:
D. Second energy law and increasing disorder: The second energy law can be stated in a number of ways:
Heat always flows spontaneously from hot (high-quality energy) to cold (low-quality energy). Energy tends to flow or change spontaneously from and concentrate and ordered form to a more dispersed and disorder form. You learned this the first time you touched a hot stove as a child!
Fuels and foods can be used only once to perform useful work. Once a piece of coal or a tank full of gasoline is burned, its high-quality potential energy is lost forever. This means that the net useful, or high-quality energy available from fossil fuels, uranium, or any concentrated energy source is even less than predicted by the first energy law:
D. Second energy law and increasing disorder: The second energy law can be stated in a number of ways:
Heat always flows spontaneously from hot (high-quality energy) to cold (low-quality energy). Energy tends to flow or change spontaneously from and concentrate and ordered form to a more dispersed and disorder form. You learned this the first time you touched a hot stove as a child!
Other examples of increasing disorder:
a) A glass falls to the floor and shatters into a more disordered state
b) A dye crystal dropped into water spontaneously dissolves and spreads (dispersed and disordered state throughout the solution)
c) When you die, the highly ordered array of molecules decays to many smaller molecules that become dispersed throughout the environment
d) Smoke from a smokestack and exhaust from an auto disperse spontaneously to a more random or disordered state in the atmosphere
e) Pollutants dumped into a river spread spontaneously throughout the water. Until we discovered that the atmosphere and water systems could be overloaded, we assumed that such spontaneous dilution solve the problem of pollution.
a) A glass falls to the floor and shatters into a more disordered state
b) A dye crystal dropped into water spontaneously dissolves and spreads (dispersed and disordered state throughout the solution)
c) When you die, the highly ordered array of molecules decays to many smaller molecules that become dispersed throughout the environment
d) Smoke from a smokestack and exhaust from an auto disperse spontaneously to a more random or disordered state in the atmosphere
e) Pollutants dumped into a river spread spontaneously throughout the water. Until we discovered that the atmosphere and water systems could be overloaded, we assumed that such spontaneous dilution solve the problem of pollution.
In all systems, it is not enough just to look at the isolated system’s entropy. You must look at changes in disorder in both the system and its environment or surroundings. All organisms and ecosystems are not closed systems, but open systems. Look at your own body. To form and preserve its highly ordered arrangement of molecules and its organized network of chemical reactions, you must continually obtain high-quality energy and raw materials from your surroundings.
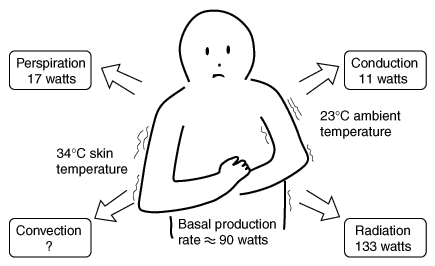
This means that disorder is created in the environment: primarily in the form of low-quality heat. Just think of all the disorder in the form of heat that is added to the environment just to keep you alive. Planting, growing, processing, and cooking foods all require energy inputs that add heat to the environment. Indeed, your body continuously gives off heat equal to that from a 100-watt light bulb, explaining why a closed room full of people gets warm.
This does not even count the enormous amounts of disorder added to the environment when concentrated deposits of minerals and fuels are extracted from the earth and burned or dispersed to heat the buildings you use, to transport you, and to make roads, clothes, and shelter.
Thus, all forms of life are tiny pockets of order maintained by creating a sea of disorder around themselves. The primary characteristic of modern industrial society is an every increasing use or flow of high-quality energy to maintain the order in our bodies and the pockets of order we call civilization. As a result, today’s industrialized nations are creating more environmental disorder than any society in human history.
This does not even count the enormous amounts of disorder added to the environment when concentrated deposits of minerals and fuels are extracted from the earth and burned or dispersed to heat the buildings you use, to transport you, and to make roads, clothes, and shelter.
Thus, all forms of life are tiny pockets of order maintained by creating a sea of disorder around themselves. The primary characteristic of modern industrial society is an every increasing use or flow of high-quality energy to maintain the order in our bodies and the pockets of order we call civilization. As a result, today’s industrialized nations are creating more environmental disorder than any society in human history.
In considering the system and surroundings as a whole, scientists have found that there is always a net increase in disorder with any spontaneous chemical or physical change, either in the environment, in the system, or in both. Thus we must modify our original hypothesis to include our surroundings: Any system and its surroundings as a whole spontaneously tend toward increasing randomness or disorder, or increasing entropy.
Scientists frequently use the concept of entropy, a measure of relative randomness or disorder. A random system has high entropy (high disorder), and an orderly system has low entropy (low disorder). Using this concept, we can state the second energy law also means that the world gets more disordered each day.
2nd law also states that systems tend to go to higher states of disorder (entropy) (your room example) where the useful amount of high quality energy decreases.
Life depends on physical world, usually living things expend energy to work against physical forces and to maintain order in their bodies. A bird flies by expending energy from metabolizing food, but this expenditure of energy may bring more energy to the bird (i.e., finding and capturing more food, escaping enemies). It appears that life violates the 2nd law (this argument has been used to support creationism – living things are too intricate to have evolved, also, if evolution appear to bring on more complex organisms, isn’t this violating the 2nd law). No, not really. You see the increase in disorder of the environment through time. The sun powers the maintenance of complexity by providing us and the earth with a constant supply of high-quality energy (i.e., neither the earth nor living things are truly closed systems). In addition, all living things die, and thus consequently follow the 2nd law eventually (the dead body decomposes to CO2, water, and various nutrients).
Sunlight is the most important energy source to earth. It warms the earth, the atmosphere and the oceans, sunlight energy drives the oceans and air circulation patterns, and sunlight energy is captured by plants and converted to chemical energy in food.
E. These laws and environmental concerns
E. These laws and environmental concerns
The law of conservation of matter and the first and second laws of energy give us keys for understanding and dealing with environmental problems.
The “throwaway” society found in most industrial countries is based on using more and more of the earth’s matter and energy resources at a faster and faster rate. The earth receives a constant flow of energy from the sun, but for all practical purposes little matter enters or leaves the earth [meteors in; space craft components out]. We have all of the matter that we will ever have.
The “throwaway” society found in most industrial countries is based on using more and more of the earth’s matter and energy resources at a faster and faster rate. The earth receives a constant flow of energy from the sun, but for all practical purposes little matter enters or leaves the earth [meteors in; space craft components out]. We have all of the matter that we will ever have.
Some people and scientists (note: the last phrase does not suggest that scientists are humanoid aliens) would point out that not all is bad:
a) Technology can help us stretch our supplies of matter resources and perhaps find substitutes
b) Some argue that because of the matter and energy laws, sooner or later we must face up to the finiteness of the earth’s resource supplies
c) Others talk of infinite supplies or resources on earth or propose schemes to get new supplies of energy and matter from space
d) Others agree that resource supplies on earth are finite but argue that we are not close enough to exceeding any built-in limits to worry about them
a) Technology can help us stretch our supplies of matter resources and perhaps find substitutes
b) Some argue that because of the matter and energy laws, sooner or later we must face up to the finiteness of the earth’s resource supplies
c) Others talk of infinite supplies or resources on earth or propose schemes to get new supplies of energy and matter from space
d) Others agree that resource supplies on earth are finite but argue that we are not close enough to exceeding any built-in limits to worry about them
Some say we should become a matter-recycling society so that economic growth can continue indefinitely without depleting material resources. But there is a catch to recycling. In using resources such as iron, we dig up concentrated deposits of iron ore. Then, we disperse this concentrated iron over much of the globe as it is fashioned into useful products, discarded, or changed into other chemical substances. To recycle, we must collect it, transport it to central recycling centers or steel mills, and melt/purify it so that it can be used again. This is where the two energy laws come in. Recycling matter always requires high-quality energy. In the long run, a recycling society based on indefinitely increasing economic growth must have an essentially inexhaustible and affordable supply of high-quality energy. And high-quality energy, unlike matter, can never be recycled.
“Don’t we have an essentially infinite supply of solar energy flowing into the earth?” Sunlight reaching the earth is high-quality energy, but the quantity reaching a particular area of the earth’s surface each minute or hour is low and is nonexistent at night. Using solar energy to provide hot water and to heat a house to moderate temperatures makes sense. However, using solar energy to provide the high temperatures needed to melt metals or to produce electricity in a solar power plant may not make sense. In these cases, solar energy must be collected and concentrated to provide the necessary high temperatures, which is quite expensive.
One way to lessen the problem involves the development and widespread use of solar photovoltaic cells that convert sunlight directly to electricity in one simple nonpolluting step.
If present research increases the efficiency of such cells and decreases their cost, we could be covering entire roofs of houses and building with rows of these cells to provide all the electricity we need. Such a development could, in a fairly short time, make large, centralized electric power plants in the world obsolete. Mass production and transportation of solar cells would require energy and matter resources. But most of the matter needed for solar cells would come from silicon, one of the most abundant chemicals on earth.
One way to lessen the problem involves the development and widespread use of solar photovoltaic cells that convert sunlight directly to electricity in one simple nonpolluting step.
If present research increases the efficiency of such cells and decreases their cost, we could be covering entire roofs of houses and building with rows of these cells to provide all the electricity we need. Such a development could, in a fairly short time, make large, centralized electric power plants in the world obsolete. Mass production and transportation of solar cells would require energy and matter resources. But most of the matter needed for solar cells would come from silicon, one of the most abundant chemicals on earth.
Even with such a technological advance, the second energy law tells us that as we use more and more energy to transform matter into products and then recycle these products, the disorder in the environment will increase. Thus the second energy law tells us that the more we try to order, or “conquer” the earth, the greater the disorder we put into the environment. We will always attempt to order the environment to some extent for our benefit, but the second energy law helps us understand that we should be so with ecological wisdom, care, and restraint.
Why do some think that we can avoid the effects of the second law ofenergy? Part of the problem is ignorance. Many have not heard of the second law of thermodynamics, let alone understand its significance. In addition, this law has a cumulative rather than individual effect. You accept the law of gravity because it limits you and everyone else on a personal level. However, although your individual activities automatically increase the disorder in the environment, this effect seems small and insignificant. But the cumulative impact of the disorder-producing activities of billions of individuals trying to convert more and more of the world’s resources to trash and low-quality heat as fast as possible eventually can have a large, negative impact on the local and global life-support systems.
As we find more sources of energy, it is argued that more people on this planet will benefit from cheaper, reliable, abundant energy for their wants and needs. However, if we bypass one limiting factor, we run into another. Some scientists worry that if we use more energy, it just allows humankind to further accelerate the use and abuse of out other resources, greatly increasing the problems of scarcity and pollution. Think of the huge impact if any living person lived the same lifestyle as the people in North America (we are about 5% of the human population, but we use 20 to 90% or more of most resources, and we contribute more to environmental degradation and pollution than many other countries).